-
×
 Rosewater Facial Spray
1 × $14.00
Rosewater Facial Spray
1 × $14.00 -
×
 High Potency Vitamin C Serum
1 × $32.00
High Potency Vitamin C Serum
1 × $32.00
Azulene
« Back to Glossary Index
Named after the Spanish word azul for blue, azulene dazzles with an indigo hue that looks almost Photoshopped. The color isn’t cosmetic smoke and mirrors; it arises from a non-alternant aromatic hydrocarbon structure featuring fused five- and seven-membered rings that absorb light in the orange region, reflecting blue. Distilled from German chamomile (Matricaria chamomilla) or yarrow (Achillea millefolium), azulene emerges as a minor but ultra-potent fraction in the essential-oil matrix, often under one percent by weight yet responsible for most of the plant’s anti-inflammatory charm.
Lab assays show that azulene suppresses histamine release from mast cells and inhibits the cyclooxygenase pathway, which means it can quiet both immediate redness and deeper prostaglandin-driven swelling. In a hamster ear-edema model, topical azulene outperformed 0.5-percent hydrocortisone at reducing inflammatory thickness after UVB insult. That steroid-rivaling data explains why azulene has long featured in post-waxing lotions, after-shave balms, and even tattoo aftercare creams.
Because its coloration is intense, formulators dose azulene sparingly – typically 0.02 to 0.1 percent – just enough to confer a spa-worthy turquoise tint without staining towels. To forestall fading, they package products in cobalt glass or opaque tubes and buffer the formula around neutral pH (extremes of acidity fade azulene to olive). Synergy with bisabolol, chamomile’s other star molecule, heightens the calming cascade, offering a one-two punch against flushing rosacea.
For end users, azulene-laced ampoules can rescue skin after an over-zealous peel or sunburn: a cooling veil that diminishes heat within minutes and speeds the return to baseline over hours. Makeup artists adore azulene primers because the blue counteracts sallow or ruddy undertones, pre-correcting the canvas. Sensitive souls who recoil at menthol or alcohol in traditional aftershaves find azulene a gentler path to post-blade peace.
Azulene (Wikipedia)
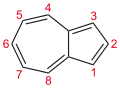
Azulene is an aromatic organic compound and an isomer of naphthalene. Naphthalene is colourless, whereas azulene is dark blue. The compound is named after its colour, as "azul" is Spanish for blue. Two terpenoids, vetivazulene (4,8-dimethyl-2-isopropylazulene) and guaiazulene (1,4-dimethyl-7-isopropylazulene), that feature the azulene skeleton are found in nature as constituents of pigments in mushrooms, guaiac wood oil, and some marine invertebrates.
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Azulene | |||
| Systematic IUPAC name
Bicyclo[5.3.0]decapentaene | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.005.449 | ||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C10H8 | |||
| Molar mass | 128.174 g·mol−1 | ||
| Melting point | 99 to 100 °C (210 to 212 °F; 372 to 373 K) | ||
| Boiling point | 242 °C (468 °F; 515 K) | ||
| −98.5·10−6 cm3/mol | |||
| Thermochemistry | |||
Std enthalpy of
combustion (ΔcH⦵298) |
−1266.5 kcal/mol | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Azulene has a long history, dating back to the 15th century as the azure-blue chromophore obtained by steam distillation of German chamomile. The chromophore was discovered in yarrow and wormwood and named in 1863 by Septimus Piesse. Its structure was first reported by Lavoslav Ružička, followed by its organic synthesis in 1937 by Placidus Plattner.